We are probably all familiar with multiple health problems that affront the world today, such as food and water contamination in some countries, malnourishment in others, obesity in developed countries, and in much more recent circumstances, the emergence of SARS- CoVi-2, the coronavirus which causes COVID. However, one of the rising problems that is sometimes overlooked is the problem of Antibiotic Resistance in infections and disease.
So to start off, what is Antibiotic Resistance? Antibiotic Resistance, or AR, is the trend in bacterial (not viral, never viral) species to better survive a given antibiotic over prolonged exposure. Now, there are two ways primarily that AR genes can emerge in a population; they can emerge by random mutations or by Horizontal Gene Transfer (HGT).
Some people may believe that Antibiotic Resistance is entirely human caused, and that resistance did not occur until after humans began to implement antibiotic use in medical treatment. However, we have learned that this is not the case. Most antibiotic compounds are actually millions of years old, invented by mother nature and good old random mutations. For example, the fungus Penicillium chrysogenum, produces the antibiotic penicillin as a natural defense mechanism against bacterial attacks (more on penicillin here) Us humans merely discovered these qualities, and used them to our advantage. However, with every population that interacts in nature, there is often a co-evolutionary relationship. Some bacteria over time, in response to the emergence of penicillin, developed enzymes which could destroy penicillin, allowing them to survive against the fungus’ defenses. This battle between bacteria and fungi, in creating new antibiotics, and then new resistance genes, has been going on ling before humans were ever on the planet.
Yet, there is another way in which resistance genes emerge in a population: through Horizontal Gene Transfer (HGT). HGT is the process by which bacteria share their genes either by accident, or intentionally in order to respond to environmental stressors. This is completed in three different ways: transformation, conjugation, and transduction. For the discussion of AR and human actions that contribute to its increase, HGT is a much more important aspect of bacteria because of how fast it can occur.
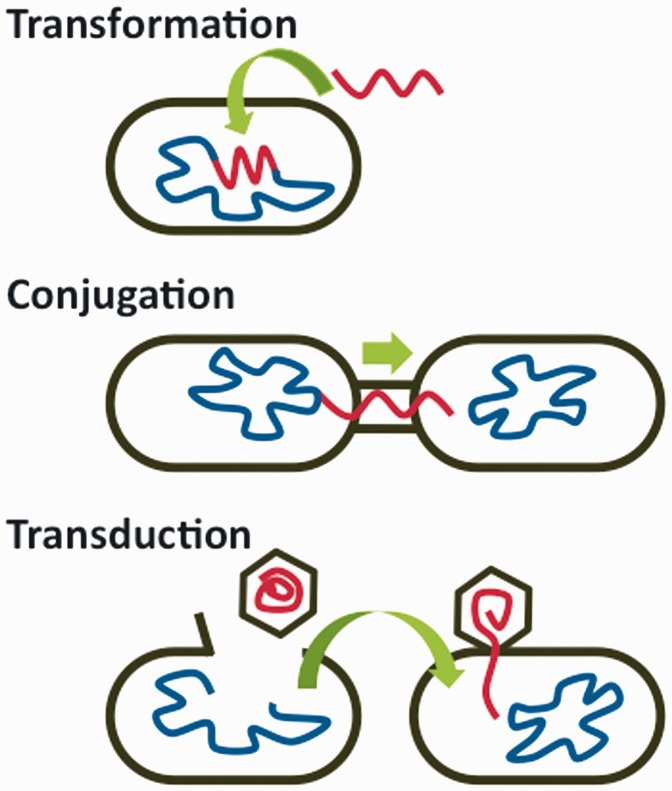
Transformation occurs when a bacteria takes up genes for DNA that is floating around in the environment. Conjugation occurs when bacteria directly share genes through a protein channel in their membranes. Transduction occurs when a bacteria accidentally receives the DNA of another bacteria from a virus called a bacteriophage.
Now that you are versed in antibiotic resistance and the mechanisms that cause it, we are now going to discuss how humans have been contributing to its increase. One of these ways is through medical practices. For example, some of the more recent research that I have found indicated that in treating a patient with antibiotics, the composition of their microbiomes, or the resident bacteria in their gut, can be significantly impacted to increase resistance. Inside the gut, there are thousands of species of bacteria, all with different genes and different traits that help them survive. They all inhabit their own ecological niches in the gut, and are all limited for the consumption of nutrients by other species. By treating a patient with antibiotics, the more susceptible species are eliminated, which removes competition, and allows the stronger, resistant species to survive and dominate the gut.
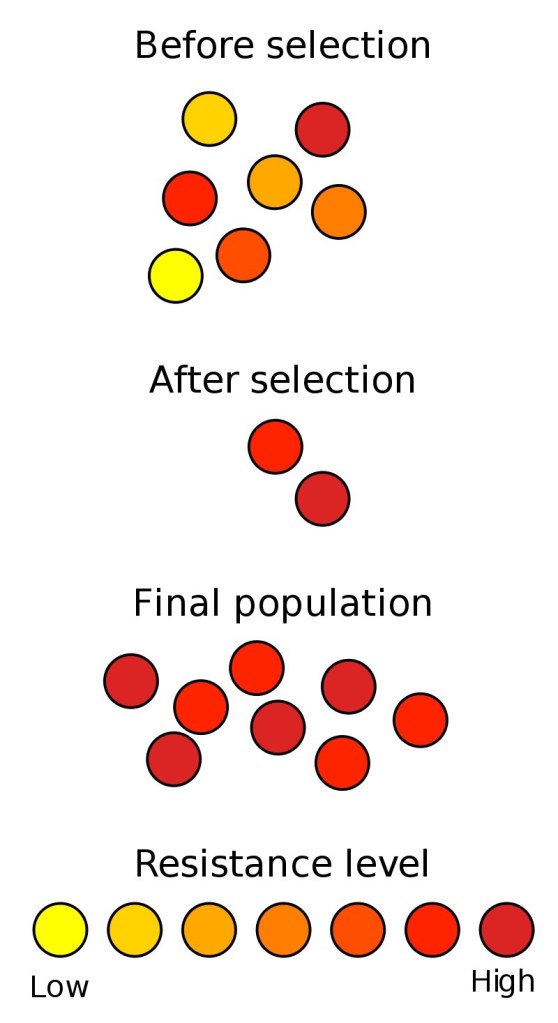
Some more research I found indicated that treatment with antibiotics also directly stimulated bacteria to share genes in an attempt to survive. This research found that antibiotic treatment causes the expression of “SOS genes,” which are a set of genes which are used in emergency scenarios to increase survivability. They found that treatment with some broad spectrum antibiotics caused gram negative bacteria to increase their conjugative gene transfer, which the bacteria did in hopes of finding genes which could nullify an antibiotic attack. This finding is particularly troubling, because it indicated that treatment with antibiotics may cause a normally non threatening bacteria residing in the gut to give its resistance genes to a potentially deadly bacteria in these conditions, creating a newer tough strain of disease causing bacteria.
Another way in which research is indicating that humans are causing increased AR is through agriculture, and particularly, CAFO’s (Concentrated Animal Feeding Operations). Since about the 1950’s, humans have been prophylactically treating animals with antibiotics in order to increase their health, and increase their yields. There is a major incentive to give livestock antibiotics because they have shown to grow animals bigger on less feed, which typically lowers consumer price and increases efficiency. However, there is a downside to this. Broad spectrum use of antibiotics in animals actually increases the possibility that more resistant and deadly recombinants of human pathogenic bacteria will arise.
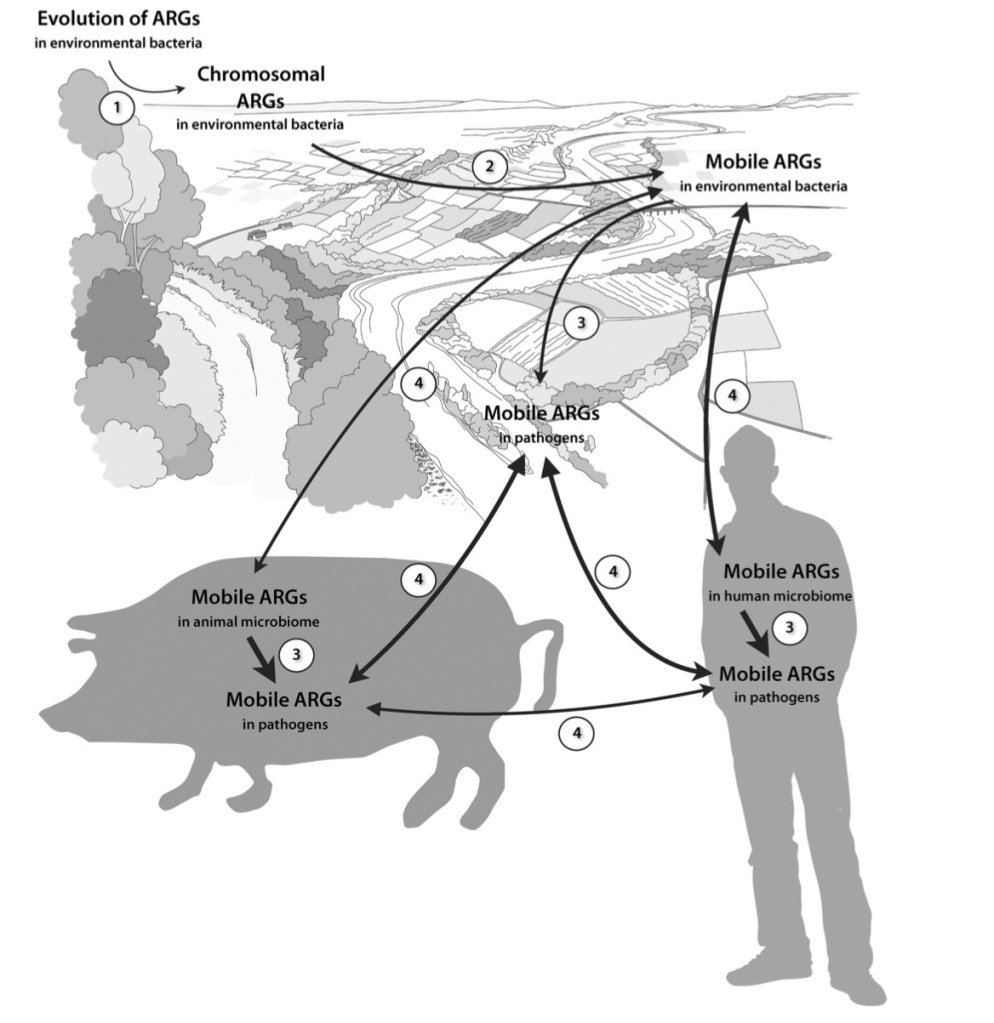
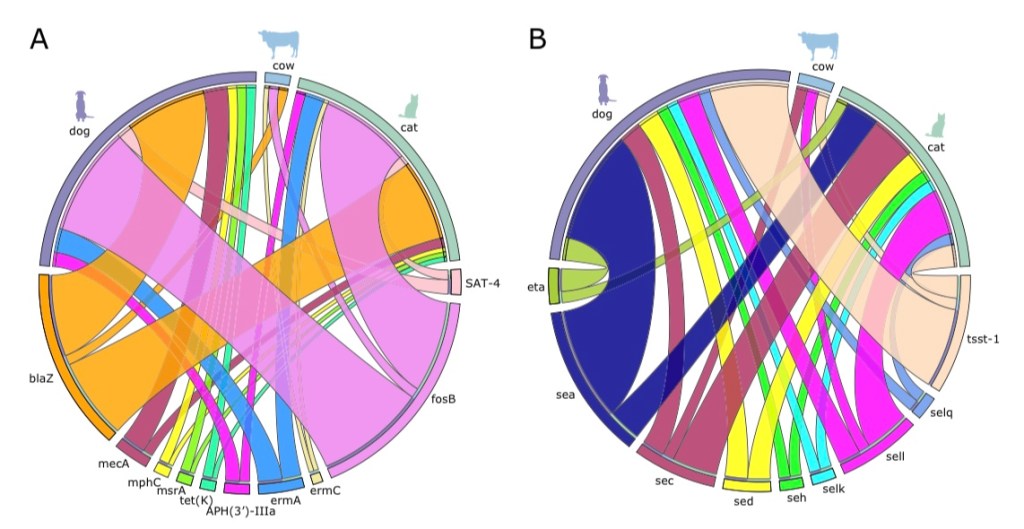
But wait? How could treating animals pose a danger to humans for human disease? Well, as it turns out, many bacteria which live in and infect humans also commonly exist in domestic livestock. These “reservoirs,” often serve as the primary sources for new emergent strains in endemic outbreaks. For example, a recent study I looked at tracked resistance genes and virulence genes across strains of Staphylococcus aureus in the northeastern United States across multiple vertebrate and it was found that there was “widespread gene sharing” among species in close proximity to humans (particularly among dogs, cats, and cattle.) Other research I looked at has indicated that has indicated that aspects of agriculture, such as runoff and leeching from feces infected water, or dust particles from livestock, can also serve as vectors for resistance genes.
Across the board, human activities have been attributed to the general increase in prevalence of antibiotic resistant outbreaks of infectious bacteria. For example, there is no better case of deadly antibiotic resistant pathogens in modern society than the infamous Methicillin-Resistant Staphylococcus aureus, or MRSA for short. A recently conducted observational study I examined found that within the past decade or so, the number of hospital associated MRSA infections has more than doubled across the United States. Luckily the mortality rate attributed to this pathogen has been stagnating, and symptoms have been lessening to those of minor skin infections due to the development of better antibiotic treatments (Malacidins). Still, numbers of infections related to antibiotic resistance continue to rise globally.
Another study I looked at found that Salmonella ssp. endemic outbreaks related to resistance across both the developed and developing world have been increasing. This study showed that there was a divide in how resistance grows between the developed and developing world, tracing an increase in non typhoidal resistant Salmonella outbreaks in the developed world, and and increase in typhoidal resistant Salmonella outbreaks in the developed world (particularly in Africa). There are many factors that contribute to this of course, but one of the interesting remarks about this recent epidemiological research is how it correlates the differing use of antibiotics in agriculture in the developed and developing world.
Currently in the developing world, there is a widespread call to severely limit the extensive prophylactic use of antibiotics in agriculture due to its acknowledged outcomes. For example, in the United States the FDA outwardly claims that widespread use of antibiotics “can contribute to the emergence of antimicrobial resistance in bacteria that may be transferred to humans.” They are currently working on trying to limit antibiotic use in livestock to veterinary oversight post-infection.
Turning over to the developing world however, we are beginning to see a sharp increase in the antibiotic use in agriculture, as such countries are using their benefits to increase yields to feed their populations and increase their GDPs. As a result of this trend, epidemiological evidence suggests that the highest rates of emergence of AR will be found in the developing world.
Due to all of these trends, we are expecting to see antibiotic resistance increase dramatically in the future. It is expected that the number of deaths within the next 2-3 decades will rise north of 10,000,000 annually across the world.
There is still hope though. Ongoing research is finding promise in isolation of antibiotics from soil samples, as I have stated in one of my more previous blogs. For us as readers, it is important that we are informed about the dangers of misusing antibiotics, and that we are aware of sanitation in our hospitals and food.
To sum op the majority of the information above, I have created an infographic of some important facts about antibiotic resistance.
